Abstract
Vitamin D deficiency is common in many societies and causes rickets and non-skeletal disorders in children. There are no published data on vitamin D deficiency in Afghanistan. We, therefore, investigated the prevalence and associated factors of vitamin D deficiency in Afghan school children in Kandahar City, Afghanistan. This cross-sectional analytical study was conducted from September 2022 to April 2023 in 510 primary school students aged 6–15 years from six randomly selected schools. Data were analyzed by using descriptive statistics, Chi-square test, and multivariate logistic regression. Of the 510 enrolled children, 54.3% were boys and 91.8% were poor. The mean serum 25(OH)D concentration was 9.3 ng/mL. Vitamin D deficiency (< 20 ng/mL) was detected in 436/510 (85.5%) children that was severe in 267/510 (52.4%). By logistic regression analysis, independent factors for vitamin D deficiency were: (i) older age group 11–15 vs. 6 − 10 years, adjusted odds ratio (AOR) 2.8 (95% confidence interval 1.2–6.2), (ii) poverty AOR 2.0 (1.0–4.3), (iii) not doing outdoor physical activity AOR 4.8 (2.8–8.1), and (iv) daily sunlight exposure < 60 min AOR 2.2 (1.3–3.7). Although Kandahar is very sunny throughout the year, vitamin D deficiency is highly prevalent among school boys and girls, placing them at great risk of vitamin D-deficient rickets. More work is needed to define the country-wide prevalence of vitamin D deficiency to inform robust strategies of vitamin D supplementation, the provision of vitamin D-fortified food to the school children in Kandahar City and health education programs that can be conducted with the help of international organizations.
Similar content being viewed by others
Introduction
Vitamin D is a fat-soluble vitamin, mostly responsible for the health and growth of bones by enhancing the absorption of calcium, magnesium, and phosphate1. The main source of vitamin D is exposure to sunlight resulting in the conversion of 7-dehydrocholesterol to vitamin D3 (cholecalciferol) in the skin2, which undergoes two metabolic steps: C-25 hydroxylation in the liver to 25 hydroxyD3 (25-hydroxycholecalciferol) and then to 1,25 dihydroxyD3 (1,25 dihydroxycholecalciferol in the kidneys. Vitamin D in food is in the form of vitamin D2 (ergocalciferol) and D3 and foods rich in vitamin D3 include oily fish like salmon, trout, and tuna; cod liver oil is a particularly rich source3. Other food sources are egg yolk, fortified cereals, and fortified milk. Vitamin D2 is found in plants and mushrooms are a rich source of vitamin D2. Vitamin D2 undergoes the same metabolism as vitamin D3 and collectively they are known as 25(OH)- and 1,25(OH)2D. 1,25(OH)2D is the active form of vitamin D that promotes the active absorption of calcium in the small intestine and this is the form that is measured in serum.
The concentration of 25(OH)D reveals the overall status of vitamin D in the body and concentrations < 50 nmol/L (20 ng/mL) are considered diagnostic of vitamin D deficiency1. In the absence of renal dysfunction, common causes of vitamin D deficiency include poor diet, limited sun exposure, increased skin pigmentation, use of sunblock creams, and changes in latitude1,2. In children, severe vitamin D deficiency might cause rickets4 while moderate vitamin D deficiency negatively affects bone acquisition5,6. the prevalence of vitamin D deficiency varies by geographical region. Based on the reports of national surveys and other studies, the prevalence of vitamin D deficiency was ~ 40% in Europe (< 50 nmol/L)7, 23–30% in the United States of America8,9, 34% in Africa (< 50 nmol/L)10, 30–90% in the Middle East11, 20% in Australia12, and 56% in China13.
A study was conducted among 107 socioeconomically deprived children under five years of age in Kabul, the capital city of Afghanistan. The median concentration of serum 25(OH)D was 5 ng/mL (range 2–24 ng/mL) with no statistically significant difference between boys and girls. The serum levels of 25(OH)D were reported to be < 8 ng/mL, 8–15 ng/mL, and > 15 ng/mL in 73.1%, 23.1%, and 3.8% of the children, respectively14. In another study of 308 adolescents in Kabul, 61.0% (188/308) had vitamin D deficiency; 26.3% (81/308) had mild or moderate vitamin D deficiency and 34.7% (107/308) had severe vitamin D deficiency15. A hospital-based study was conducted in Kabul on 4000 children, aged 1 month to 18 years, who visited the pediatric department. The prevalence of vitamin D deficiency was 41.3% (1650/4000) with 22.9% (917/4000) having severe vitamin D deficiency16.
Very few studies have been conducted in Kandahar involving children17,18,19,20,21,22 and none has investigated vitamin D deficiency. In a personal communication with pediatricians in Kandahar City, the issue of increased vitamin D deficiency among Kandahar children was raised. Although there is plenty of sunshine in Kandahar throughout the year, pediatricians still observe signs and symptoms of vitamin D deficiency among school-age children. To the best of our knowledge, there has never been a study from Afghanistan that has examined the prevalence of and associated risk factors for vitamin D deficiency in primary school children. We, therefore, set out to conduct such a study in Kandahar and report the results herein.
Materials and methods
Study design and study area
This was a school-based, cross-sectional study conducted during a seven-month period, from September 2022–April 2023, in Kandahar, the second largest city of Afghanistan, situated in south-west Afghanistan (coordinates: 31° 37′ 12″ N 65° 42′ 57″ E) at an altitude of 1,010 m above sea level. The population of Kandahar is a little over 614,000 people and there are 145 schools located in the city.
Study population and sample size calculation
The study population of this study was composed of schoolchildren aged 6–15 years who were permanent residents of Kandahar. Children were excluded if they had diseases of the skin, liver, kidneys, and intestine, that might affect the absorption or metabolism of vitamin D, and those who refused (either child or guardian) to participate in the study.
The sample size was calculated using the software of Epi Info version 7.2.2.6 (CDC, Atlanta, Georgia, USA). We added a 20% non-response rate. The selection of a 20% non-response rate was based on the rule already set for this study by the quality control committee of Kandahar University Research Center. So, our sample size was 565 children.
Among the 565 children, four (0.7%) had skin diseases (all five children had psoriasis), two (0.4%) had liver diseases (both had jaundice and positive history of hepatitis B), one (0.2%) had kidney disease (this patient had nephrotic syndrome), and guardians of 48 (8.5%) children did not agree to participate in the study. So, we collected data from 510 children.
Sample collection and laboratory procedures
Six government schools (three for boys and three for girls) were selected using a lottery method of randomization. From each school, children in grades 1–5 were also randomly selected by lottery. Socio-demographic, anthropometric, and laboratory data of the enrolled children were collected using researcher-made questionnaires in the Pashto and Dari languages. Data were collected on paper case record forms by well-trained investigators who were trained by Kandahar University Faculty of Medicine researchers for three days. One day consisted of classroom training followed by two days of practical training in the selected schools.
Anthropometric data, like height and weight, were collected from all the enrolled children. They stood barefooted against a wall and their height was measured using a stadiometer with an accuracy of 0.1 cm. All children were weighed in light clothes using a digital balance of 0.1 kg. For quality control, approximately 10% of the measurements were randomly selected and measured by an experienced researcher who was blinded by the previous measurement results; the selection of 10% was based on the rule already set by the quality control committee of Kandahar University Research Center.
A total of 5 mL of blood was taken for serum 25(OH)D levels and stored at room temperature before transfer to the laboratory within one hour. Samples were centrifuged and serum 25(OH)D was measured using a chemiluminescence immunoassay (Biomerieux, Mini VIDAS, Paris, France). All the lab examinations were performed following the guidelines and regulations of the Afghanistan Ministry of Public Health. Vitamin D status was classified based on the serum level of 25(OH) D (see definitions below). All methods and procedures were performed in accordance with the relevant guidelines and regulations.
Ethical considerations
Ethical approval was obtained from the Ethics Committee of Kandahar University (code number KDRU-EC-2022.25) as well as permission from the Kandahar Province Education Department. Before the data collection and following an explanation of the study, written informed consent and assent were obtained freely from all the parents/guardians and children. For the data collection, only the initials of the study participants were used on the case record forms and sample labeling but all collected data were coded and de-identified for data entry.
Data entry and analysis
All the data were double entered and cleaned in Microsoft Excel 2021 and then imported into the Statistical Package for the Social Sciences (SPSS) version 22 (Chicago, IL, USA) for statistical analysis. To summarize demographic characteristics, descriptive analyses were used, such as frequency, percentage, mean, and standard deviation (SD). The chi-square test (using crude odds ratio [COR]) was used to assess the binary association between categorical variables and students’ t-test or nonparametric equivalent were used for analyzing continuous data. All statistically significant variables in the univariate analyses were assessed for independence in a multivariate logistic regression (using adjusted odds ratio [AOR]) to determine the risk factors associated with vitamin D deficiency; factors examined included age, sex, and economic status. All analyses were two-sided and a p-value < 0.05 was considered statistically significant.
Definitions
Cut-off levels of 25(OH)D for vitamin D status23
-
Vitamin D sufficiency: ≥30 ng/mL.
-
Vitamin D insufficiency: 20–29.9 ng/mL.
-
Vitamin D mild/moderate deficiency: 10–<20 ng/mL.
-
Vitamin D severe deficiency: <10 ng/mL.
Poverty: Family income < 170 Afghanis (< 1.90 USD) per person per day24.
Physical activity: School children and their parents/guardians were asked about the number of days in one week they did physical activity (walking, recreational activity, or sports) and were into acceptable (physical activity ≥ 3 days per week) or unacceptable (physical activity < 3 days per week).
Fitzpatrick classification of skin types25
-
Type I: Very white or very fair skin color.
-
Type II: White or fair skin color.
-
Type III: Medium white to light brown skin color.
-
Type IV: Olive or moderate brown skin color.
-
Type V: Brown to dark brown skin color.
-
Type VI: Very dark brown to black skin color.
Results
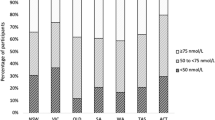
Of 565 children assessed, 510 were enrolled in autumn (32.0%), winter (34.7%), and spring (33.3%); boys and girls were roughly equal. The mean (SD) age, height, and weight were 8.9 (2.3) years, 127.5 (9.4) cm, and 20.3 (3.1) kg, respectively. Overall, 401 children were aged 6–10 years and the majority ethnic group was Pashtun (Table 1). The mean (SD) level of serum 25(OH)D in all children was 9.3 (6.5) ng/mL and 436/510 (85.5%) had vitamin D deficiency [25(OH)D value < 20 ng/mL]; 267/510 (52.4%) suffered from severe (< 10 ng/mL) vitamin D deficiency (Fig. 1; Table 2).
The vast majority, 91.8% (468/510), of children were poor, 67.5% (344/510) did little outdoor physical activity, and only 17.6% (90/510) ate fish at least once every two weeks. Most of the study participants had either Fitzpatrick Type III (297/510 [58.2%]) or Type IV (198/510 [38.8%]) skin color while 32 (6.3%) girls only used topical sunscreen (Table 2). None of the children were taking vitamin D supplements or eating vitamin D-fortified food.
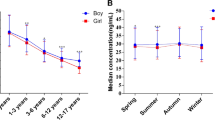
Table 3 shows the status of vitamin D in school children during the three seasons of the year. Data for summer were not available due to the school holidays. A statistically significant difference was not observed during the three seasons with sufficient (normal), insufficient, mild/moderate, and severe deficiency of vitamin D.
Univariate analysis (Table 4) using Chi-square test showed that the statistically significant risk factors associated with vitamin D deficiency in school children were: (i) age group 11–15 years (COR 2.9, 95% CI 1.3–6.6, p = 0.007), (ii) poverty (COR 2.3, 95% CI 1.1–4.8, p = 0.025), (iii) not doing outdoor physical activity (COR 4.6, 95% CI 2.8–7.8, and p = < 0.001), (iv) daily sunlight exposure < 60 min (COR 2.0, 95% CI 1.2–3.3, p = 0.006), and (v) not eating fish at least once every two weeks (COR 1.8, 95% CI 1.0–3.2, p = 0.049).
By logistic regression analysis, the statistically significant independent risk factors associated were: (i) age group 11–15 years (AOR 2.8, 95% CI 1.2–6.2, and p = 0.014), (ii) poverty (AOR 2.0, 95% CI 1.0–4.3, and p-value 0.049), (iii) not doing outdoor physical activity (AOR 4.8, 95% CI 2.8–8.1, and p-value < 0.001), and (iv) daily sunlight exposure < 60 min (AOR 2.2, 95% CI 1.3–3.7, and p-value 0.005).
Discussion
In this school-based cross-sectional analytical study, we studied 510 primary school children for a period of seven months in six schools in Kandahar City, Afghanistan. We found a high prevalence of vitamin D deficiency in these school children and the associated risk factors were older age (11–15 years), decreased sunlight exposure, not doing outdoor physical activity, and poverty.
The prevalence of vitamin D deficiency in this study was a staggering 85%. Similar vitamin D deficiency prevalence rates have been reported from Tunisia, ~ 85% of 225 healthy children aged 7–16 years26, and Iran, ~ 80% in 477 healthy school children aged 9–18 years27. However, even higher rates have been observed in Saudi Arabia, 100% in 331 healthy urban children aged 6–17 years28, Malaysia, ~ 93% in 1361 healthy school students with a mean age of almost 13 years29, and India, 92% in 713 healthy school children aged 10–14 years30.
By contrast, lower but highly variable prevalence rates of vitamin D deficiency were been reported: no vitamin D deficiency in 103 healthy children (mean age 6.7 years)31, 3.6% in 225 healthy Tunisian children (mean age 9.5 years)32, 11.5% of 200 healthy primary school children, aged 9–11 years, from Egypt)33, 18% among 4,558 healthy American (USA) children aged 1–11 years34, almost 27% in 470 healthy Italian children aged 5–10 years35, ~ 30% in 435 healthy Algerian children aged 5–15 years36, ~ 35% in 1,102 healthy British children, aged 4–18 years37, ~ 40% in 99 healthy South Africans, aged 11–20 years38, 44% in 174 healthy Ethiopian students, aged 11–18 years39, and in Finland, 71% of 195 healthy school children and adolescents age 7–19 years5.
In all the above-mentioned studies, vitamin D deficiency was defined as a 25(OH)D concentration of < 50 nmol/L or < 20 ng/mL. The marked variation in vitamin D deficiency prevalence rates could be due to differences in various factors such as testing methods, latitudes, season of testing, age range tested, and nutritional habits. In North America and some European countries, vitamin D supplementation and fortification of foods with vitamin D is the main source of vitamin D40,41. However, this is not the case in Afghanistan where the dietary intake of vitamin D is poor, as fish and liver are scarce and relatively expensive42. Although better-off families can afford to eat a range of food, often they do not with one study reporting low food diversity scores throughout Afghanistan with an average of 15 (range 7–20)42. Furthermore, vitamin D-rich foods such as fish, liver, and eggs are believed to be “hot” or “hard” and are not given to children14.
In Kandahar, the cheapest way to obtain vitamin D is exposure to the sun. There is plenty of sunshine in Kandahar throughout the year and mean sun hours reported throughout the year range from 248 h in February to a high of 365 h in July for an annual mean of 329 h, which is higher than the mean sun hours for other large cities in Afghanistan43. Despite the long sun hours, the high prevalence of vitamin D deficiency in this study could be due to skin color (mostly brown), which negatively affects the synthesis of vitamin D by the skin, and traditional dress that covers the arms and legs. Therefore, the best options for the prevention of vitamin D deficiency in Afghan children could be health education regarding exposure to sunlight, as well as the provision of vitamin D supplements and vitamin D-fortified food.
In this study, being an older child (aged 11–15 years) was a risk factor for vitamin D deficiency, consistent with results from Iran (477 children aged 9–18 years)27, India (404 girls aged 6–18 years)44, Lebanon (179, aged 10–16 years)45, Turkey (51560, aged 0–18 years)46, Algeria (435, aged 5–15 years)36, and Great Britain (1102, aged 4–18 years)37. Compared to older children, younger children tend to be more physically active, spend more time outdoors (school and playground), and have a healthier diet enriched by dairy foods given to them by parents47,48. Puberty was identified as an independent factor in Iran due to the increased demand for vitamin D at this stage of development27. However, the Iranian study suggested more research was needed because further work is needed to explain the reasons for the decrease in serum 25(OH)D among older children since this appears to be independent of the effects of physical activity, pubertal status, sun exposure, or obesity27.
Two other key risk factors we identified that have also been reported widely across a range of studies from low to high-income countries were low outdoor physical activity and poverty27,36,37,44,45,49. Encouraging greater physical activity in prepubertal Caucasian children in Australia revealed that physical activity and sunlight exposure had a positive association with bone mass measurements and mineralization50 and should be included in health promotion activities. Tackling poverty is a greater challenge; foods that are rich in vitamin D, such as fish and liver, are expensive in Afghanistan42.
Being a girl was not a risk factor for vitamin D deficiency in this study and this accords with several other studies27,37 but thus has been reported as a factor in children from Malaysia (mean age ~ 13 years)29, Lebanon (10–16 years)45, Italy (5–10 years)35, and Finland (7–19 years)5. One factor in some societies may be the dress code for girls and women who are obliged to cover themselves; moreover, girls are more likely than boys to avoid the sun for cosmetic reasons.
Strengths and limitations
This is the first study to examine vitamin D status among school children from Afghanistan. The sample size was large and covered six schools that were randomly selected throughout Kandahar City, allowing a degree of confidence in generalizing our results to Kandahar. The study had several limitations. First, it was only conducted in school-age children in Kandahar City; therefore, our results cannot be generalized to the whole country, different age groups, rural populations, and urban populations with different climates. Although we were not able to collect data during the summer, this is probably a minor limitation, given the high sunlight hours in Kandahar. The information for certain relied on memory; so, recall bias cannot be ruled out. Finally, this was a cross-sectional study, and the 25(OH)D concentration was only measured once; nevertheless, given the high rate of vitamin deficiency in these children, it is unlikely this had an impact on our results.
Conclusion
We found a very high rate of vitamin D deficiency (> 85%) and more than half had severe deficiency even though Kandahar has abundant sunshine throughout the year. The main risk factor was poor physical activity followed by older age with similar risks for poverty and reduced sunshine exposure. Addressing vitamin D deficiency in Kandahar is not just about immediate health outcomes but ensuring a healthier future for the next generation of Afghanistan and avoiding the damaging effects of rickets.
Our results should be important and helpful to clinicians, nutritionists, public health officials, policymakers, parents, and government and non-government organizations who are engaged in the health and well-being of children in Afghanistan. We recommend that the Afghanistan Ministry of Public Health, with the help of international organizations (such as WFP, WHO, and UNICEF), develop strategies for enhancing nutritional education in schools, conducting periodic campaigns of vitamin D supplementation (aimed mostly at school children), encouraging and providing vitamin D-rich foods (e.g., oil, milk, and wheat flour), and promoting outdoor physical activities. Additional studies should be conducted on school children of rural areas in Kandahar province. Large-scale nationwide multicenter studies should be conducted across the age spectrum and including urban and rural populations to gain a better understanding of the challenge posed by vitamin D deficiency.
Data availability
All the data and materials related to this study are available on request from the corresponding author.
Abbreviations
- 25:
-
Hydroxyvitamin D25(OH)D
- AOR:
-
Adjusted odds ratio
- BMI:
-
Basal mass index
- CI:
-
Confidence interval
- COR:
-
Crude odds ratio
- ng:
-
Nanogram
- mL:
-
Milliliter
- SD:
-
Standard deviation
- SPSS:
-
Statistical Package for the Social Sciences
- UNICEF:
-
United Nations Children’s Fund
- USD:
-
United States Dollar
- WFP:
-
World Food Program
- WHO:
-
World Health Organization
References
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357 (3), 266–281 (2007).
Holick, M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96 (7), 1911–1930 (2011).
Holick, M. F. & Chen, T. C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 87 (4), 1080S–1086S (2008).
Holick, M. F. Resurrection of vitamin D deficiency and rickets. J. Clin. Invest. 116 (8), 2062–2072 (2006).
Pekkinen, M., Viljakainen, H., Saarnio, E., Lamberg-Allardt, C. & Mäkitie, O. Vitamin D is a major determinant of bone mineral density at school age. PLoS One 7 (7), e40090 (2012).
Lehtonen-Veromaa, M. K. M. et al. Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: A 3-y prospective study. Am. J. Clin. Nutr. 76 (6), 1446–1453 (2002).
Cashman, K. D. et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 103 (4), 1033–1044 (2016).
Herrick, K. A. et al. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 110 (1), 150–157 (2019).
Schleicher, R. L. et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am. J. Clin. Nutr. 104 (2), 454–461 (2016).
Mogire, R. M. et al. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health 8 (1), e134–142 (2020).
Lips, P. et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 180 (4), P23–54 (2019).
Malacova, E. et al. Prevalence and predictors of vitamin D deficiency in a nationally representative sample of adults participating in the 2011–2013 Australian Health Survey. Br. J. Nutr. 121 (8), 894–904 (2019).
Yu, S. et al. The high prevalence of hypovitaminosis D in China: A multicenter vitamin D status survey. Med. (Baltim). 94 (8), e585 (2015).
Manaseki-Holland, S., Zulf Mughal, M., Bhutta, Z. & Qasem Shams, M. Vitamin D status of socio-economically deprived children in Kabul, Afghanistan. Int. J. Vitam. Nutr. Res. 78 (1), 16–20 (2008).
Azizi, S. & Tariq, T. M. Vitamin D deficiency among Afghan adolescents in Kabul. J. Coll. Physicians Surg. Pak. 29 (11), 1072–1077 (2019).
Sediqi, M., Mansoor, A. & Mangal, M. Prevalence of hypovitaminosis D among children and adolescents of Kabul: A descriptive cross-sectional study. BMC Pediatr. 23 (1), 52 (2023).
Rahimi, B. A., Mohammadi, E., Stanikzai, M. H. & Wasiq, A. W. Determinants of exclusive breastfeeding practices in Kandahar, Afghanistan: A cross-sectional analytical study. Int. J. Pediatr. 8 (4), 11125–11140 (2020).
Rahimi, B. A., Ishaq, N., Mudaser, G. M. & Taylor, W. R. Outcome of acute bacterial meningitis among children in Kandahar, Afghanistan: A prospective observational cohort study. PloS One 17 (4), e0265487 (2022).
Rahimi, B. A. et al. Prevalence and associated risk factors of soil-transmitted helminth infections in Kandahar, Afghanistan. BMC Infect. Dis. 22 (1), 361 (2022).
Rahimi, B. A. et al. Treatment outcomes and risk factors of death in childhood tuberculous meningitis in Kandahar, Afghanistan: A prospective observational cohort study. Trans. R. Soc. Trop. Med. Hyg. 116 (12), 1181–1190 (2022).
Stanikzai, M. H., Zakir, S., Ishaq, N. & Rahimi, B. A. Prevalence of anemia and its associated factors among children under 5 years of age attending a comprehensive healthcare facility in Kandahar City, Afghanistan. Indian J. Public. Health 66 (3), 378 (2022).
Rahimi, B. A. et al. Prevalence of soil-transmitted helminths and associated risk factors among primary school children in Kandahar, Afghanistan: A cross-sectional analytical study. PLoS Negl. Trop. Dis. 17 (9), e0011614 (2023).
Saggese, G. et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 44 (1), 51 (2018).
The World Bank. World Bank 2022 Fact Sheet: An Adjustment to Global Poverty Lines. https://www.worldbank.org/en/news/factsheet/2022/05/02/fact-sheet-an-adjustment-to-global-poverty-lines (2024).
Fitzpatrick, T. B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 124 (6), 869–871 (1988).
Bezrati, I. et al. Vitamin D inadequacy is widespread in Tunisian active boys and is related to diet but not to adiposity or insulin resistance. Libyan J. Med. 11, 31258 (2016).
Saki, F., Dabbaghmanesh, M. H., Omrani, G. R. & Bakhshayeshkaram, M. Vitamin D deficiency and its associated risk factors in children and adolescents in southern Iran. Public. Health Nutr. 20 (10), 1851–1856 (2017).
Al-Musharaf, S. et al. Vitamin D deficiency and calcium intake in reference to increased body mass index in children and adolescents. Eur. J. Pediatr. 171 (7), 1081–1086 (2012).
Al-Sadat, N. et al. Vitamin D deficiency in Malaysian adolescents aged 13 years: Findings from the Malaysian Health and Adolescents Longitudinal Research Team study (MyHeARTs). BMJ Open. 6 (8), e010689 (2016).
Khadgawat, R. et al. Impact of vitamin D fortified milk supplementation on vitamin D status of healthy school children aged 10–14 years. Osteoporos. Int. 24 (8), 2335–2343 (2013).
Omole, K. O., Kuti, B. P., Oyelami, O. A., Adegbola, A. J. & Omole, J. O. Serum vitamin D profile of Nigerian children with asthma: Association with asthma severity and control. Pediatr. Pulmonol. 53 (5), 544–551 (2018).
Maalmi, H. et al. Association of vitamin D receptor gene polymorphisms with susceptibility to asthma in Tunisian children: A case control study. Hum. Immunol. 74 (2), 234–240 (2013).
Shady, M. M. A., Youssef, M. M., Shehata, M. A., El-Din, E. M. S. & ElMalt, H. A. Association of serum 25-hydroxyvitamin D with life style and dietary factors in Egyptian prepubescent children. Open. Access. Maced J. Med. Sci. 3 (1), 80–84 (2015).
Mansbach, J. M., Ginde, A. A. & Camargo, C. A. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics 124 (5), 1404–1410 (2009).
Galeazzi, T. et al. Vitamin D status in healthy Italian school-age children: A single-center cross-sectional study. Ital. J. Pediatr. 49 (1), 27 (2023).
Djennane, M. et al. Vitamin D status of schoolchildren in Northern Algeria, seasonal variations and determinants of vitamin D deficiency. Osteoporos. Int. 25 (5), 1493–1502 (2014).
Absoud, M., Cummins, C., Lim, M. J., Wassmer, E. & Shaw, N. Prevalence and predictors of vitamin D insufficiency in children: A Great Britain population based study. PLoS One 6 (7), e22179 (2011).
Poopedi, M. A., Norris, S. A., Micklesfield, L. K. & Pettifor, J. M. Does vitamin D status track through adolescence? Am. J. Clin. Nutr. 102 (5), 1025–1029 (2015).
Wakayo, T., Belachew, T., Vatanparast, H. & Whiting, S. J. Vitamin D deficiency and its predictors in a country with thirteen months of sunshine: The case of school children in central Ethiopia. PLoS One 10 (3), e0120963 (2015).
Calvo, M. S., Whiting, S. J. & Barton, C. N. Vitamin D fortification in the United States and Canada: Current status and data needs. Am. J. Clin. Nutr. 80 (6 Suppl), 1710S–1716S (2004).
Spiro, A., Buttriss, J. L. & Vitamin, D. An overview of vitamin D status and intake in Europe. Nutr. Bull. 39 (4), 322–350 (2014).
Johnecheck, W. A. & Holland, D. E. Nutritional status in postconflict Afghanistan: Evidence from the national surveillance system pilot and national risk and vulnerability assessment. Food Nutr. Bull. 28 (1), 3–17 (2007).
World Weather Online. Kandahar Annual Weather Averages - Kandahar, Afghanistan. (2024). https://www.worldweatheronline.com/kandahar-weather-averages/kandahar/af.aspx Accessed 23 Jun 2024.
Puri, S. et al. Vitamin D status of apparently healthy schoolgirls from two different socioeconomic strata in Delhi: Relation to nutrition and lifestyle. Br. J. Nutr. 99 (4), 876–882 (2008).
El-Hajj Fuleihan, G. et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics 107 (4), E53 (2001).
Sari, E., Çoban, G., Oztek, F. & Acoglu, E. The status of vitamin D among children aged 0 to 18 Years. J. Pediatr. Res. 8 (4), 438–443 (2021).
Choi, H. S. et al. Vitamin D insufficiency in Korea–A greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J. Clin. Endocrinol. Metab. 96 (3), 643–651 (2011).
Ginde, A. A., Liu, M. C. & Camargo, C. A. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch. Intern. Med. 169 (6), 626–632 (2009).
Jones, G., Dwyer, T., Hynes, K. L., Parameswaran, V. & Greenaway, T. M. Vitamin D insufficiency in adolescent males in Southern Tasmania: Prevalence, determinants, and relationship to bone turnover markers. Osteoporos. Int. 16 (6), 636–641 (2005).
Jones, G. & Dwyer, T. Bone mass in prepubertal children: Gender differences and the role of physical activity and sunlight exposure. J. Clin. Endocrinol. Metab. 83 (12), 4274–4279 (1998).
Acknowledgements
We are sincerely thankful of the authorities of Kandahar University, Kandahar Directorate of Education, and all the enrolled schools. We are also thankful of all the data collectors and our study participants along with their guardians.
Funding
This study did not receive any specific funding. W. R. Taylor is part-funded by Wellcome under grant 220211. For Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
Conceptualisation: BAR, AAK, AU, WAK, WRT.Data curation: BAR, AAK, WAK, JAR.Formal analysis: BAR, AAK.Funding acquisition: No fundingInvestigation: BAR, AAK, WAK, JAR.Methodology: BAR, AU.Project administration: BAR, AAK.Resources: BAR, AAK, WAK, JAR.Software: BAR, AAK, AU, AQB.Supervision: BAR, AAK. Validation: BAR, AU.Visualisation: BAR, WAK.Writing – original draft: BAR, AAK.Writing – review & editing: BAR, AAK, AU, WAK, AQB, JAR, WRT.All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical clearance was obtained from the Ethics Committee of Kandahar University (code number KDRU-EC-2022.25) and permission from Kandahar Province Education Department authorities.
Consent to participate
Prior to the data collection, written informed consent from parents, and written informed assent from study participants were obtained with the help of authorities. Interviews of participants using a predesigned questionnaire were conducted and blood samples were done as per standard operating procedures. For data collection, only patients’ initials were used. Information on the participants would not be disclosed. Prior to entering into the computer for analysis, the collected data were coded and de-identified.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahimi, B.A., Khalid, A.A., Usmani, A. et al. Prevalence and risk factors of vitamin D deficiency among Afghan primary school children. Sci Rep 14, 27167 (2024). https://doi.org/10.1038/s41598-024-77330-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77330-9